ABSTRACT
Thyroid nodules and thyroid cancer are common in elderly patients and demonstrate age-specific prevalence, malignancy risk, and clinical behavior. Improved risk stratification by ultrasound characteristics and molecular testing of thyroid nodules pre-operatively has reduced the need for diagnostic surgery in many individuals. In patients with differentiated thyroid cancer, total thyroidectomy and radioactive iodine followed by thyroid stimulating hormone (TSH) suppression remain the mainstays of therapy. However, newer approaches of active surveillance and thyroid lobectomy have expanded treatment options for patients with low risk differentiated thyroid cancer. Co-morbid conditions and patient preference should inform management of thyroid nodules and thyroid cancer in the elderly, with particular attention to the risks of surgery and medication adverse effects. Furthermore, the mechanisms underlying the distinct clinical behavior of thyroid cancer found in older patients, including the drivers of more advanced stage at presentation, higher recurrence risk, and greater mortality, remain poorly understood. Patients with advanced thyroid cancer may benefit from recently developed targeted and immune therapies.
THYROID NODULES IN THE ELDERLY
Thyroid nodules are common in clinical practice and present unique management issues in elderly patients. The reported prevalence of thyroid nodules in iodine sufficient regions is 1-6% as detected by palpation, or as high as 19-68% when detected by ultrasound imaging (1-5). Evaluation of thyroid nodules is increasingly a concern for general internists and endocrinologists in the context of an aging population, increased use of imaging in clinical practice, and rising obesity.
Thyroid nodules are more frequent in elderly patients, with a linear increase with age in both the presence of nodules and the absolute number of nodules per patient (6). Approximately 50% of individuals aged 65 years have thyroid nodules detected by ultrasonography (7). A cross-sectional survey of asymptomatic adults in Germany using ultrasonography to detect thyroid nodules demonstrated an even higher prevalence of 80% in women and 74% in men over 60 years old (4). In a prospective study of 6,391 patients referred for thyroid nodules at a large academic center, Kwong et al. showed a linear increase in the number of thyroid nodules per patient with age, rising from an average of 1.55 nodules ≥1 cm in patients age 20–29 years old to a mean of 2.21 nodules ≥1 cm in patients ≥70 years old, demonstrating a 1.6% annual increased risk for multinodularity (6).
Another potential contributor to this rising prevalence of thyroid nodules is the increased use of high-frequency ultrasound, CT, and MR imaging in routine clinical care, leading to the detection of asymptomatic, or incidental, thyroid nodules (4,5,7,8). Lastly, changes in population demographics over time, specifically increased rates of obesity, may contribute. Data from several ethnically diverse cohorts has identified parameters independently associated with the development of thyroid nodules, including obesity, female sex, radiation exposure, iodine deficiency, and smoking. These factors should be noted when evaluating elderly patients for potential thyroid nodules (9).
THYROID NODULE EVALUATION
Once identified, thyroid nodules should be evaluated to determine appropriate management. The differential diagnosis of thyroid nodularity includes benign and malignant solitary nodules, multinodular goiter, autonomous functioning nodules, cysts, and inflammation or thyroiditis (10). Nodules causing thyroid dysfunction, compressive symptoms, or harboring malignancy require attention.
In the presence of biochemical and/or clinical signs of hyperthyroidism, a radioiodine uptake and scan should be pursued to distinguish autonomous nodules. Adjunctive data to support a diagnosis of inflammation or autoimmune destruction may include thyroid autoantibodies [anti-thyroid peroxidase (TPO) and anti-thyroglobulin (Tg)]. In addition, the presence of thyroid stimulating immunoglobulins suggest a diagnosis of Graves’ disease in the presence of goiter (11).
Nodules without associated thyroid function abnormalities should be further evaluated to determine or exclude the presence of cancer. Guidelines from the American Thyroid Association summarize the management of non-functional thyroid nodules based upon imaging and patient characteristics (12). A general approach to evaluation of thyroid nodules is shown in Figure 1.
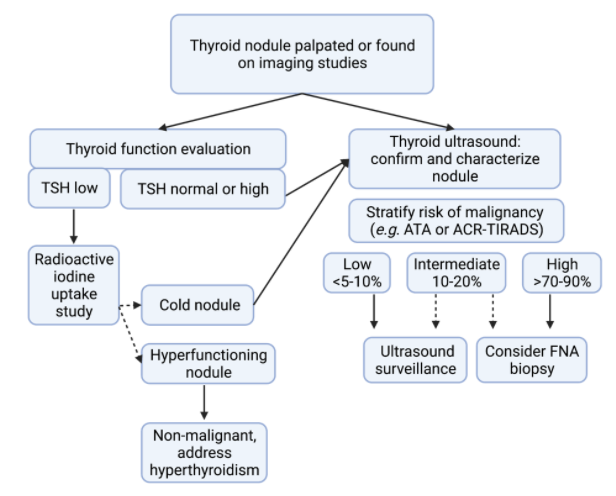
Figure 1. Approach to evaluation of thyroid nodules. Evaluation always includes measurement of thyroid function tests, including thyroid stimulating hormone (TSH), and thyroid ultrasound. If hyperthyroidism is present, a thyroid uptake to exclude an autonomous functioning nodule (i.e. hyperfunctioning thyroid follicular tissue producing thyroid hormone excess). Cold nodules refer to nodules without autonomous production of thyroid hormone. Systems developed for malignancy risk stratification of thyroid nodules on ultrasound include the 2015 American Thyroid Association guidelines (ATA) and the American College of Radiology (ACR-TIRADS). Thyroid fine needle aspiration (FNA) biopsy should be considered for nodules with intermediate or high risk of malignancy based upon size and patient specific factors.
Thyroid ultrasound is the most important imaging modality in the assessment of thyroid nodules. Multiple systems have been developed to stratify thyroid nodules by their malignancy risk based upon ultrasound findings and provide recommendations for FNA biopsy (12-15). A systematic review and meta-analysis of eight studies including 13,092 thyroid nodules compared the diagnostic performance of four commonly used ultrasound-based risk stratification systems: the American College of Radiology Thyroid Imaging and Reporting System (ACR-TIRADS), the American Thyroid Association (ATA), the Korean Thyroid Imaging and Reporting System (K-TIRADS) and European Thyroid Imaging and Reporting System (EU-TIRADS) (12-16). This analysis found that the pooled rate of unnecessary FNA biopsies (i.e., those with a benign cytology result) was significantly lower with ACR-TIRADS (25%) when compared to ATA (51%, p<0.001) and K-TIRADS (55%, p<0.001), and not statistically different from EU-TIRADS (38%, p=0.087) (16). The diagnostic odds ratios among these four systems was similar (16). Features of thyroid nodules commonly associated with a higher risk of malignancy across these systems include solid and hypoechogenic appearance, irregular margins, microcalcifications, taller than wide shape, and evidence of extrathyroidal extension (12-15). Iso- or hyperechogenic appearance, smooth margins, and spongiform or partially cystic composition are features less associated with malignancy (12-15). Based upon ultrasound evaluation, nodules with highest risk for malignancy are recommended to have further evaluation by thyroid biopsy.
THYROID NODULE BIOPSY
Fine needle aspiration (FNA) biopsy is the recommended modality for sampling thyroid nodules. Cytology specimens collected by FNA are classified traditionally by the Bethesda System for Reporting Thyroid Cytopathology (17) across six categories: (i) non-diagnostic or unsatisfactory; (ii) benign; (iii) atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS); (iv) follicular neoplasm or suspicious for a follicular neoplasm; (v) suspicious for malignancy; and (vi) malignant (Table 1). The risk of malignancy determined by surgical pathology is estimated across each category and used to guide decisions about continued clinical observation or treatment with surgical resection (17).
|
Table 1. Bethesda System for Reporting Thyroid Cytopathology and Associated Estimated Risk of Malignancy. |
||||
|
Bethesda category |
Cytopathology |
Cytologic descriptions |
Malignancy risk Cancer = NIFTP Cancer ≠ NIFTP |
Typical management |
|
I |
Non-diagnostic
|
Acellular specimen Cyst fluid only Obscuring factors |
5-10% 5-10% |
Repeat FNA
|
|
Ii |
Benign
|
Benign follicular nodule Chronic lymphocytic thyroiditis Granulomatous thyroiditis |
0-3% 0-3% |
Clinical and ultrasound follow-up |
|
Iii |
Atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS) |
Atypia: Cytologic (focal nuclear changes, extensive but mild nuclear changes, atypical cyst lining cells, or ‘‘histiocytoid’’ cells) and/or architectural (predominantly microfollicles, sparsely cellular); Hurthle cells |
6-18% 10-30% |
Repeat FNA, molecular testing, or diagnostic lobectomy |
|
Iv |
Follicular neoplasm or suspicious for a follicular neoplasm
|
Follicular-patterned cases with mild nuclear changes (increased nuclear size, nuclear contour irregularity, and/or chromatin clearing), and lacking true papillae and intranuclear pseudo-inclusions |
10-40% 25-40% |
Molecular testing or diagnostic lobectomy |
|
V |
Suspicious for malignancy
|
Features suspicious for PTC, MTC, lymphoma, or other malignancy |
45-60% 50-75% |
Total thyroidectomy or lobectomy |
|
Vi |
Malignant
|
Features conclusive for malignancy: PTC (true papillae, psammoma bodies, nuclear pseudo-inclusions) MTC Poorly differentiated / ATC Non-endocrine malignancy (squamous cell, lymphoma, metastatic)
|
94-96% 97-99% |
Total thyroidectomy or lobectomy |
PTC, papillary thyroid carcinoma. MTC, medullary thyroid cancer. ATC, anaplastic thyroid cancer. NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features.
In situations of non-diagnostic FNA results or indeterminate cytology (i.e., Bethesda iii or iv), repeat FNA biopsy is recommended. Additionally, three molecular tests are now available for further cancer risk stratification and can reduce the number of thyroid surgeries performed for ultimately benign lesions (18). The ThyroSeq v3 multigenomic classifer (University of Pittsburgh Medical Center and CBL PATH, Pittsburgh, PA) is a DNA based assay that detects the presence of high-risk cancer mutations and was initially developed as a rule-in test for thyroid cancer (19). The current version, ThyroSeq v3, incorporates 112 genes associated with thyroid cancer and has a reported sensitivity of 98% and specificity of 81% for detection of thyroid cancer from FNA samples (20). The Afirma genomic sequencing classifier (GSC) (Veracyte, San Francisco, CA, USA) evaluates mRNA expression associated with benign or malignant profiles and detects thyroid cancer-associated mutations (21, 22). The Afirma GSC has a reported sensitivity of 91% (95% CI, 79-98) and a specificity of 68% (95% CI, 60-76) for thyroid cancer (21). A recent randomized clinical trial compared the diagnostic performance between the Thyroseq v3 and Afirma GSC assays in 346 patients with 427 cytologically indeterminate nodules (median age, 55 years) (18). This study found that both molecular tests showed high specificity for thyroid cancer with no significant differences in diagnostic performance, leading to 49% of patients avoiding diagnostic surgery. Lastly, the combined ThyraMIR microRNA Classifier and ThyGenX Oncogene Panel (Interpace Diagnostics, Parsippany, NJ) is a cancer rule-in test that uses multiplex PCR to identify cancer-associated gene mutations and translocations, done in tandem with evaluation of microRNA expression. The test estimated negative predictive value and positive predictive value are 94% and 74%, respectively (23). As molecular testing continues to evolve, clinicians and patients will have additional tools to aid in treatment decisions.
AGE-SPECIFIC NODULE PREVALENCE AND MALIGNANCY RISK
Several studies have specifically addressed thyroid cancer risk and nodule management across the age spectrum. Kwong et al. (6) reported the rate of malignancy in a cohort of 6,391 patients referred to a large academic center who underwent thyroid ultrasound and FNA of 12,115 nodules (all ≥1 cm). With advancing age, the prevalence of clinically relevant (>1 cm) thyroid nodules increased, whereas the risk that such nodules were malignant decreased. For patients ages 20–29, 30–39, 40–49, 50–59, 60–69, and >70 years, the cancer prevalence was 22.9, 21.8, 17.1, 13.0, 13.7, and 12.6%, respectively (p<0.001). When the malignancy rate was analyzed “per-nodule,” the youngest cohort (20–29 years) demonstrated a 14.8% malignant risk per nodule at diagnosis in comparison to 5.6% in the oldest cohort (>70 y; p<0.01). Between the ages of 20 and 60 years, each advancing year was associated with a 2.2% reduction in the relative risk that any newly evaluated thyroid nodule was malignant (OR 0.972; p<0.001), and this risk of malignancy stabilized after age 60 years. However, this study also found that despite a lower likelihood of malignancy for nodules in elderly patients, these cancers were more likely to have aggressive phenotypes (6).
Further addressing the burden and risk of thyroid nodule evaluation in older patients, Angell and colleagues analyzed a large cohort of elderly patients (age 70 years and older) who underwent thyroid nodule evaluation over a 20-year period (24). In this study, 1,129 patients over the age of 70 years with 2,527 nodules ≥1 cm were evaluated. Thyroid cancer-specific mortality was observed in 8% of thyroid cancer patients. All such patients could be recognized during initial evaluation based on the presence of invasive tumor, extensive lymph node metastases, or distant metastases. While FNA was a safe procedure in this age-group and a benign result was obtained in two-thirds of samples, FNA led to surgery in 208 patients, of whom 93 (44.7%) had benign histopathology. These data suggest that while an identifiable group of older patients are at risk for mortality from thyroid cancer warranting aggressive treatment, many patients ≥70 years old derive little benefit or are even harmed by thyroid nodule therapy.
Judicious use of FNA biopsy, improved stratification of nodule cancer risk by ultrasound characteristics, and molecular testing have improved pre-operative determination of malignancy risk in patients with thyroid nodules and reduced the need for diagnostic surgery. However, a significant number of patients who undergo thyroid nodule resection for suspicious nodules are still ultimately found to have benign lesions on surgical histopathology. Particularly in elderly patients with a greater burden of co-morbid medical disease, the risk of unnecessary thyroid surgery is an important consideration.
DIFFERENTIATED THYROID CANCER IN THE ELDERLY
While thyroid nodules are relatively common in elderly patients and the vast majority are benign (24), thyroid cancer is identified in a subset. Patients and their families are often concerned about the implications of this diagnosis and disease outcomes. Several subtypes of thyroid cancer are frequently encountered and increasing information about the underlying biology of these malignancies is now available. Most thyroid cancers are identified incidentally on imaging rather than by palpation on physical examination. Rarely, symptoms of thyroid cancer can include lymphadenopathy, hoarseness from laryngeal nerve involvement, dysphagia, airway compression from mass effect, or pain; when present, these symptoms portend more advanced disease and worse clinical prognosis (25, 26). When thyroid cancer is identified, a combination of surgical, radioactive iodine, and surveillance strategies are employed and tailored to the individual patient and disease characteristics.
Incidence and Prevalence of Thyroid Cancer
Thyroid cancer currently accounts for 2.3% of all new cancers, with an estimated 44,280 new cases in 2021, but only 0.4% of cancer deaths, in the United States annually (27). In the general population, the peak occurrence is between ages 51 and 60 years (28). Thyroid cancer is more common in women than men and among those with a family history of thyroid disease (27).
The incidence of thyroid rose over the past few decades, from an incidence of approximately 5 new cases per 100,000 persons per year in 1975 to a peak of 15 new cases per 100,000 in 2014 (27). The rate of thyroid cancer more recently has remained near 13 to 14 cases per 100,000 (27). Notably, small (<2 cm) papillary thyroid cancers account for the majority of this increase (29), and despite a much higher incidence, the death rate from thyroid cancer has remained stable (27, 30), likely reflecting greater detection of early disease associated with a good prognosis.
Classification of Thyroid Cancer
Thyroid follicular cell-derived cancer is subdivided into several histopathologic types: papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), Hurthle cell carcinoma (HCC), and poorly differentiated or anaplastic thyroid cancer (31-33). Other malignancies encountered in the thyroid include medullary thyroid cancer arising from thyroid gland C-cells (discussed below), lymphoma, and secondary metastasis of other primary cancers.
Papillary thyroid cancer (PTC) is the most common type of differentiated thyroid cancer (DTC) accounting for approximately 80 to 85% of all cases (27, 31-33). It has a bimodal frequency, with the peak incidence being in the third and sixth decades, and it affects women three times more often than men. These carcinomas arise from the thyroid follicular cells and frequently harbor BRAF V600E mutations, produce thyroglobulin (Tg), and express the sodium-iodide symporter (NIS) with resultant radio-iodine avidity (31). A history of radiation exposure increases the risk of PTC (34-36). PTC frequently spreads via the lymphatics to the regional lymph nodes, and bilateral involvement is present in approximately one-third of the cases at diagnosis. In rare cases, metastatic disease occurs in the lungs, brain, and bone (31).
Micropapillary thyroid cancer, defined as a PTC less than 1 cm in diameter and confined to the thyroid, is likely to be of minimal clinical significance (37). A prospective, observation study of papillary thyroid microcarcinoma in Japan, found that in patients less than age 40 the microcarcinoma progressed to clinical disease, (defined as significant growth, size >1.2 cm, or lymph node metastases), in contrast to those over age 60, whose disease remained static (38), suggesting that in most elderly patients these lesions can be safely observed.
Follicular thyroid cancer (FTC) is the second most common type of DTC and constitutes approximately 10 to 15% of all thyroid cancers (27, 31, 32). Risk factors include iodine deficiency and female sex (27, 39, 40). Compared to PTC, FTC less often has cervical lymph node spread but shows a predilection for vascular invasion and distant metastasis (41). Mutations of RAS, an activator of the mitogen-activated protein kinase and PI3K-AKT pathways, and rearrangements of PPAR-γ (e.g., PAX8-PPAR-γ translocation) have been implicated in the tumorigenesis of follicular adenomas and FTC (41, 42).
Hurthle cell carcinomas (HCCs) account for 5% of DTC and are characterized by an abundance of dysfunctional mitochondria (>75% of cell volume) and tendency for vascular invasion (43, 44). These malignancies are more often radio-iodine refractory and aggressive in clinical behavior. Unique genetic drivers of HCC have been reported, namely widespread loss of heterozygosity, a high burden of disruptive mutations to protein-coding and tRNA-encoding regions of the mitochondrial genome, and recurrent mutations in DAXX, TP53, NF1, CDKN1A, ARHGAP35, TERT promoter, and the RTK/RAS/AKT/ mTOR pathway (45, 46).
Anaplastic thyroid cancer is rare and discussed separately.
Variation in Histopathology and Tumor Extent by Age
Several studies have shown variance in histopathology distribution with rising age. Lin et al. (47) conducted a retrospective analysis of 204 thyroid cancer patients aged 60 years and older; 142 (70%) thyroid cancers were well differentiated and of those 68% were PTC, 30% FTC, and 2% Hurthle cell carcinoma. Fifty-nine (29%) of the thyroid cancers were poorly differentiated (39 anaplastic thyroid, 9 metastatic cancers to the thyroid, 7 lymphoma, 4 squamous cell carcinomas, and 4 without enough cells for interpretation) and 3 (2%) were medullary thyroid cancer. This pattern is significant for fewer PTC and more FTC in elderly patients, as well as more poorly-differentiated tumors.
Girardi et al. conducted a retrospective study of thyroid cancer in 596 adults from 2000-2010; their results similarly showed a lower frequency of PTC among elderly patients, with a complementary increase in the frequency of FTC, poorly differentiated and anaplastic thyroid carcinoma (28). This study also demonstrated variability in other presenting features of thyroid cancer in elderly patients (age ≥ 65 years) compared to middle-aged cohorts (25-44 years or 45-64 years); specifically, there was larger primary tumor size (median 2.1 cm for elderly versus 1.5 cm in 25-44 years and 1.1 cm in 45-64 years) and higher rates of extrathyroidal disease (mean 43% for elderly versus 25.3% in 25-44 years and 28.6% in 45-64 years) (28). Lymph node metastasis was greatest at the extremes of age (<24 and >70 years).
Similarly, Chereau et al. evaluated histopathology and extent of disease at diagnosis in elderly (65-75 years old) and very elderly (>75 years old) patients compared to younger patients in 3,835 patients treated at an academic center from 1978 to 2014 (48). These data were notable for significantly increased primary tumor size, tumor number, extra-capsular invasion, advanced TNM stage, and lymph node and distant metastasis in the very old group (48). Collectively these studies show a pattern of more widespread disease at presentation in elderly patients and a relative increase in the frequency of more aggressive histologic subtypes.
Relation of Age to Mortality and Risk of Recurrence
Numerous studies have demonstrated increased recurrence and mortality in thyroid cancer with rising age (49-54). Indeed, age is incorporated into current clinical staging systems for differentiated thyroid cancer, including the American Joint Committee on Cancer (AJCC) 8th edition (55); Metastasis, Age, Completeness of resection, Invasion, Size (MACIS) model (56); Age, Grade, Extent, Size (AGES) score; and the Age, Metastasis, Extent, Size (AMES) score (57). In all of these staging systems, advanced age is included as a risk factor predicting worse prognosis.
Historic studies by Halnan (58) and Cady et al. (59) established a positive correlation between advanced age and worse prognosis in patients with DTC, later corroborated by Ito et al. (60) in a study of 1,740 patients with PTC and by Sugino et al. (61) in 134 patients with FTC. In many of these studies, worse prognosis has been defined variably as recurrence, decreased disease- or metastasis-free survival, cause-specific mortality, and/or overall mortality. Other reports have shown that the presence of lymph node involvement and extrathyroidal extension may portend a more ominous outcome in older compared to younger patients (59, 62-64). Extrathyroidal disease in older patients increased recurrence to 67% and death rates to 60% compared to those with intrathyroidal disease, while in younger patients the relative increases were 12% and 4%, respectively (59). Additionally, the risk of death with distant metastasis is greater in older compared to younger patients (96% versus 63%) (59).
Recently, this well-accepted tenet of thyroid cancer has been modified in two important ways, namely that age likely modifies prognosis in a continuous rather than dichotomous manner and that age itself may not be as relevant to thyroid cancer behavior as the accompanying changes in accumulated cell mutations, immune senescence, and hormone changes that accompany it (65).
With the 8th edition of AJCC staging for differentiated thyroid cancer, the age threshold for increased risk was raised from 45 to 55 years, based upon several reports suggesting that this increased validity for staging (66, 67). More recent data suggest that thyroid cancer mortality and recurrence prediction is more robust when age is modeled as a continuous variable, leading some to suggest the elimination of a specific age cutoff from staging completely (65).
In a study of 3,664 patients with differentiated thyroid cancer, Ganly et al. found that disease-specific mortality increased progressively with advancing age, without a threshold age (54). Similarly, evaluation of over 30,000 patients in the SEER database by Orosco et al. demonstrated a linear association with age and thyroid cancer death (53).
A review by Haymart et al. summarizes possible biologic mechanisms underlying the clinical observations of worse thyroid cancer prognosis in the elderly (51). Briefly, mortality findings may be confounded by greater comorbid nonthyroidal diseases with older age. Higher baseline levels of thyroid-stimulating hormone (TSH) may accelerate tumor cell growth via stimulation of the TSH-receptor. If one presumes that thyroid cancers detected in elderly patients have had a longer time of subclinical growth and evolution compared to cancers detected in younger patients, then such tumors might have had greater opportunity to acquire genetic mutations facilitating cell cycle escape, loss of differentiated features (e.g., loss of sodium-iodine symporter and radioiodine avidity), and metastasis. In summary, there is significant observational evidence that older patients with thyroid cancer have worse clinical outcomes, though the precise effect of increasing age and the etiology of this distinct clinical behavior remain incompletely understood.
Treatment of Differentiated Thyroid Cancer
Historically, differentiated thyroid cancers were treated with complete surgical resection of the thyroid gland combined with thyroid hormone suppression of TSH and radioactive iodine adjuvant therapy. More recently, recognition of the overall good prognosis and low disease specific survival in patients with DTC (68) has shifted management toward greater consideration for partial thyroid surgery, reduced use of radioactive iodine (RAI) in patients with low risk of recurrent disease, and active surveillance of some cancers. Treatment strategies for progressive or metastatic disease include repeat surgery, RAI ablation, or systemic therapies (12). A general approach to the treatment of differentiated thyroid cancers is presented in Figure 2.
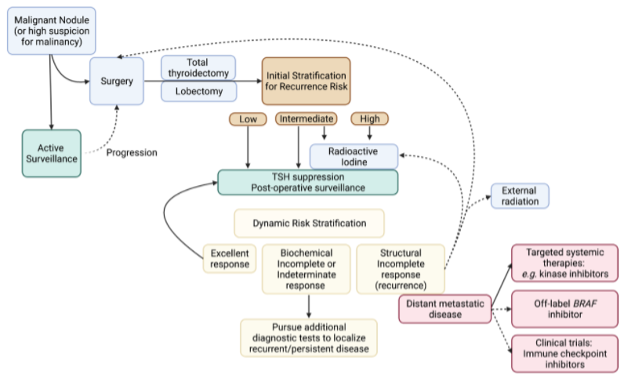
Figure 2. Approach to the treatment of differentiated thyroid cancers (DTC). RAI, radioactive iodine. TSH, thyroid stimulating hormone.
Management will be influenced by patient characteristics, such as age, comorbid conditions, and preference for invasive or conservative therapy, and modalities available at the treating center. Two competing facts in older patients must be considered in selecting appropriate therapy for each individual. First, as discussed above, thyroid cancer in elderly patients is associated with more aggressive histologic features and greater lymph node spread at diagnosis. On the other hand, thyroid-cancer related mortality remains very low and treatment-associated morbidity may pose a greater risk to the elderly patient (68, 69).
Papaleontio and colleagues recently demonstrated that competing causes of death in older patients (>65 years) with DTC contributed more to patient mortality than the underlying diagnosis of thyroid cancer (69). Among 21,509 elderly patients with thyroid cancer identified in the SEER-Medicare database, 4168 (19.4%) died of other causes versus 2644 (12.3%) died of thyroid cancer during the study period from 2000 to 2015, with median follow-up of 50 months. Specifically for DTC patients, the likelihood of dying from other causes exceeded the likelihood of dying from thyroid cancer. A competing risks hazards regression analysis showed that heart disease [HR 1.34; CI (1.25–1.44)], chronic lower respiratory disease [HR 1.25; CI (1.17–1.34)], and diabetes mellitus [HR 1.14; CI (1.06-1.21)] were associated with death from other causes. Increased probability of death from thyroid cancer was associated with non-papillary histology [e.g., FTC HR 1.29; CI (1.12-1.48), or anaplastic HR 5.51; CI (4.82-6.31)], larger tumor size [ >4cm HR 3.35; CI (2.71-4.15)], and regional or distant metastatic disease [HR 4.59; CI (3.98-5.31) and HR 12.65; CI (10.91-14.66), respectively]. Progressively advancing age was associated with an increased probability of death from both other causes and thyroid cancer. In summary, this suggests that in elderly patients a diagnosis of thyroid cancer may not be the most significant factor influencing life expectancy. A careful discussion of treatment options, including expected benefits and risks in the context of disease burden and comorbid conditions, is warranted with each patient.
SURGERY
Patients diagnosed with DTC by thyroid FNA, or with a nodule highly suspicious for malignancy, may be referred to a surgeon for thyroid resection. Total thyroidectomy aims to remove the primary tumor and normal thyroid tissue and remains the primary initial treatment for DTC (12). Thyroid lobectomy is a more limited surgery that removes only the primary tumor and ipsilateral normal thyroid lobe. Additional exploration and removal of central and lateral neck lymph nodes suspicious for cancer metastases may be done concurrently with either procedure, as guided by preoperative imaging or intraoperative findings. The decisions to pursue surgery and the extent of surgery (i.e., total thyroidectomy versus lobectomy) in an elderly patient require individual evaluation of co-morbid illnesses and life expectancy.
The most common complications of thyroidectomy include hypoparathyroidism, recurrent laryngeal nerve injury, hematoma, and wound infection; high-volume thyroid surgeons have minimal to no increase in the risk of surgical complications with increasing age (70-75). However, elderly patients are more likely to receive thyroidectomy at community and low-volume sites (76) where the rate of surgical complications may be higher. In population-based studies of thyroidectomy, which may reflect more accurately the experience of many elderly patients, increasing age is associated with longer hospital length of stay (76) and readmissions after thyroidectomy (77). In the cohort of elderly and very elderly patients studied by Chereau et al. (48), the authors found no increase in thyroidectomy-specific complications (i.e., permanent hypocalcemia and recurrent laryngeal nerve palsy) with increasing age, but did find an increase in medical complications surrounding surgery, 2.3-2.7% in those over 65 years of age compared to 0.6% in those under 65 years old.
LOBECTOMY
Thyroid lobectomy may be considered in select patients with low-risk disease (12, 70). The ATA guidelines revised in 2015 suggest that lobectomy is appropriate for DTC with a primary tumor size <4 cm and without extrathyroidal extension or clinical evidence of lymph node metastasis (12). Potential advantages of lobectomy over total thyroidectomy are lower rates of surgical complications from hypoparathyroidism and recurrent laryngeal nerve damage (78). In addition, some patients do not require thyroid hormone replacement after lobectomy to achieve the recommended low normal target TSH range of 0.5-2mIU/L (79).
On the other hand, patients who initially undergo lobectomy, but are found to have aggressive disease features (e.g.,extrathyroidal extension, lymph node metastasis) on surgical pathology, are encouraged to undergo completion thyroidectomy. This facilitates monitoring of disease with serum thyroglobulin (Tg) and treatment with adjuvant RAI (discussed below). Because elderly patients more often have aggressive disease features and higher rates of local recurrence requiring re-operation, some recommend initial total thyroidectomy in this population (73).
ACTIVE SURVEILLANCE
Although surgery is the accepted initial management for most DTC, active surveillance may be an alternative strategy to immediate surgery for an appropriately selected group of patients (80). Multiple international retrospective studies with long term follow-up suggest that many small (<1-1.5cm), well-differentiated PTCs, without evidence of extrathyroidal extension or metastases, have low rates of growth and progression (38, 81, 82). In a cohort of 291 US patients with low-risk PTC followed by serial ultrasonography, Tuttle and colleagues (74e) showed significant growth in only a minority of patients over a median follow-up of 25 months: volume increase of greater than 50% in only 36 (12%) patients, and size increase greater than 3 mm in 11 (3.8%) patients. Ito and colleagues (38) similarly showed in a cohort of 1235 Japanese patients with small PTCs followed with active ultrasonographic surveillance for a median of 75 months that, by 5 and 10 years, only 4.9% and 8% of patients experienced tumor growth of more than 3 mm, and 1.7% and 3.8% of patients experienced new lymph node metastases, respectively. Importantly, in a subset of patients who ultimately underwent thyroid surgery in this study, none were found to have distant metastases and no patient died of PTC, suggesting that delaying intervention until the time of growth or detection of lymph node spread did not adversely affect mortality. Active surveillance for low-risk PTC may avoid unwarranted surgery, surgical complications, RAI administration, and lifelong thyroid hormone replacement therapy and should be considered for appropriate patients, particularly those with reliable follow-up, high surgical risk, shorter life expectancy, or with concomitant medical issues that need to be addressed before surgery (80, 83).
RADIOACTIVE IODINE ABLATION
Based upon the extent of primary disease noted on surgical pathology (i.e., tumor size, extrathyroidal extension, lymph node and vascular spread), patients can be stratified by their risk for recurrent disease. Adjuvant therapy with radioactive iodine (I131; usual dose100-150mCi) is recommended for patients with a high risk of recurrence after total thyroidectomy, and RAI should be considered for patients with an intermediate risk of recurrence (12). An analysis of 21,870 patients with intermediate-risk PTC found that adjuvant RAI therapy was associated with a 29% reduced risk of death overall with clear benefit in those over 65 years of age (84). RAI may also be used as a treatment modality in patients with persistent or recurrent RAI-avid disease who are not surgical candidates, usually requiring doses of 150mCi or higher. Finally, lower doses of RAI (30mCi) may be used to ablate remnant normal thyroid tissue and improve the utility of serum Tg tumor marker monitoring even in patients with a lower risk of recurrence. Two multicenter studies showed that an ablative dose of 30 mCi (1.1 MBq) I131 was as effective as 100 mCi (3.7 MBq); both doses were 90% effective for ablation of residual thyroid tissue (85, 86). A long-term follow-up of one of these studies (median 4.5 years) showed that the radioiodine dose selected for remnant ablation did not affect recurrence rate (87).
Treatment benefits of RAI should be weighed against side effects. The adverse effects of RAI therapy are increasingly recognized and include transient neck pain and swelling, decreased fertility, dry mouth and eyes, and secondary malignancy and are correlated with higher I131 doses (88).
In patients initially treated with lobectomy or active surveillance for low-risk DTC, surgical removal of remaining normal thyroid tissue is recommended prior to RAI use.
THYROID STIMULATING HORMONE (TSH) SUPPRESSION
Following surgery, and RAIA if indicated, patients are treated with thyroid hormone, usually with a dose of levothyroxine that suppresses serum TSH to subnormal levels. Several special considerations for the goals of thyroid hormone therapy following thyroid cancer arise in elderly patients.
Thyroid hormone replacement is titrated to levels sufficient to suppress pituitary secretion of TSH, which is considered a growth-promoting factor for follicular cell-derived thyroid cancers. Revised guidelines from the ATA (12) suggest individualized targets for TSH suppression in thyroid cancer, generally targeting a low to low-normal range TSH. Greater TSH suppression in more aggressive disease is balanced with greater cardiac and bone complications in elderly patients.
Older patients are more likely to have co-morbid cardiac disease, including arrhythmias, coronary artery disease, and heart failure, which can place them at increased risk for complications from thyroid hormone excess. A population-based study of patients taking levothyroxine for any cause, found a significantly higher risk of cardiac arrhythmias [HR 1.6 (1.10–2.33)] and cardiovascular admission or death [1.37 (1.17–1.60)] in those with a suppressed serum TSH (≤0.03 mU/L) compared to those with TSH in the normal reference interval (89). Notably, increased cardiovascular risk was not observed in patients with a low but not fully suppressed TSH (TSH 0.04 – 0.4 mU/L). Specifically, in thyroid cancer patients treated with levothyroxine with modestly suppressed TSH (mean TSH <0.35 mU/L), atrial fibrillation was common (17.5% prevalence) in those patients ≥60 years old (89).
Longstanding hyperthyroidism is associated with osteoporotic fractures and loss of bone mineral density. Specifically, post-menopausal women (≥65yo) with suppressed TSH levels (0.1 mU/L) due to endogenous or exogenous thyroid hormone had significantly higher rates of new hip (OR 3.6, 95% CI 1.0-12.9) and vertebral fractures (OR 4.5, 95% CI 1.3 -15.6) compared to comparable women with normal TSH levels over a 3.7 years follow-up (90). In adult patients on levothyroxine therapy, a suppressed TSH (≤0.03 mU/L) was associated with a two-fold increase in risk [HR 2.02 (1.55–2.62)] of new osteoporotic fracture compared to similar patients treated with levothyroxine with a TSH maintained in the normal reference interval (89). Studies evaluating thyroid cancer patients are limited in outcome evaluation of bone mineral density (BMD) rather than fracture incidence, but generally support similar conclusions regarding lower BMD with suppressive-dose levothyroxine therapy (91-93). In elderly patients receiving TSH-suppression therapy, dual-emission X-ray absorptiometry (DEXA) monitoring of BMD should be considered based upon age and other risk factors for osteoporosis. There are no guidelines to suggest the optimal interval for DEXA screening; osteoporosis once identified should be treated using standard therapies (such as bisphosphonates or RANKL inhibitor) unless otherwise contraindicated (94).
Peripheral metabolism of thyroid hormone and clearance decreases with advanced age so that a lower medication dose is needed to achieve comparable serum levels (95, 96). Levothyroxine therapy is complicated further by polypharmacy in elderly patients, where commonly prescribed medications (e.g., calcium, iron) can decrease gut absorption of levothyroxine (97) or change drug metabolism (e.g., rifampicin, phenytoin, carbamazepine, amiodarone) (98). In summary, as suggested by society guidelines (12), TSH goals in thyroid cancer should be individualized and re-evaluated over time.
POSTOPERATIVE SURVEILLANCE
Recommended follow-up of DTC includes biochemical surveillance with measurement of serum thyroglobulin (Tg) and Tg antibody (Tg Ab) concentrations and structural surveillance with neck ultrasonography at clinically appropriate intervals (12).
Tg is a thyroid-specific protein that can be measured in blood to monitor for the presence of thyroid cancer, as well as remnant normal thyroid tissue. As such, serum Tg is used as a tumor marker for follicular cell-derived thyroid cancers, including PTC, FTC, and Hurthle cell thyroid cancer. Serum Tg levels are initially checked 4-6 weeks after total thyroidectomy, and then at intervals of 6-12 months (12). The trends of serum Tg overtime are most informative, with a rising Tg concerning for disease recurrence. While Tg levels are usually monitored in the context of a suppressed TSH, a stimulated Tg measurement may provide a more sensitive evaluation for persistent or recurrent disease (99). Finally, the measurement of serum Tg is confounded by the presence of Tg antibodies (Tg Ab), which occurs in approximately 20% of patients with DTC and can mask recurrent or persistent disease by causing falsely low or undetectable serum Tg levels (100). Evidence from retrospective studies suggests that increasing TgAb levels (measured using validated assays), compared with stable or decreasing titers, can be used as a surrogate tumor marker in these patients (100, 101). A suppressed Tg level >0.2 ng/mL after total thyroidectomy and RAI, a stimulated Tg level >2- 5 ng/mL, a rising Tg level, or the persistence of Tg antibodies, is concerning for further evaluation (12, 100). A persistently elevated (>0.2ng/mL Tg with TSH suppression or >2ng/mL stimulated Tg) or rising serum Tg or Tg Ab level should prompt concern for persistent or recurrent disease (12).
In patients who have not received RAI after thyroidectomy, residual normal thyroid tissue may contribute to a higher baseline Tg level after thyroidectomy. In these patients, a suppressed Tg >1ng/mL or rising Tg level should prompt concern for disease recurrence. Similarly, because of the significant volume of normal thyroid tissue remaining in patients who have undergone thyroid lobectomy, the optimal use of Tg in these patients remains uncertain.
DYNAMIC RISK STRATIFICATION
The process by which serum Tg (or Tg Ab) levels and imaging surveillance data are combined to make an evolving assessment of disease status in DTC patients is called dynamic risk stratification (12, 102). Patients are classified across the spectrum from no biochemical or structural evidence of disease to definite persistent or recurrent thyroid cancer.
Patients with reassuring or low risk continue on the current regimen of tumor surveillance or are relaxed to a more conservative approach. In contrast, patients in whom DRS shows an increasing risk of thyroid cancer recurrence or progression are recommended to undergo further diagnostic evaluations to localize disease and/or additional treatment. In addition, levothyroxine therapy may be adjusted for greater TSH suppression and the interval of monitoring with serum tumor markers and imaging may be shortened.
Specifically, patients with no biochemical or structural evidence of disease are deemed to have an “excellent response” (12, 102). TSH suppression is relaxed to the low normal range (0.5-2mIU/L) to mitigate long term adverse effects of iatrogenic hyperthyroidism and annual tumor marker surveillance with or without thyroid ultrasound usual. A “biochemical incomplete” response indicates persistent abnormal Tg values or increasing TgAb levels in the absence of localizable disease (12, 102). An “indeterminate response” is defined as nonspecific biochemical or structural findings that cannot be confidently classified as representing malignant disease (12, 102). Patients with a “biochemical incomplete’ or “indeterminate response” have a TSH goal of 0.1 - 0.5mIU/L. In patients with clear recurrent or persistent disease on imaging and elevated Tg or TgAb serum markers are classified as structural incomplete response” (12, 102). TSH is maintained suppressed below 0.1mIU/L. In these patients, and some with “incomplete biochemical” or “indeterminate response,” additional evaluation with neck ultrasonography, whole-body RAI scanning, and/or PET/CT depending on level of clinical suspicion, is recommended to localize residual thyroid tissue/cancer. Identification of abnormal lymph nodes or tumor mass can then be evaluated for possible further treatment with RAI, surgery, or targeted therapy.
SYSTEMIC THERAPY
Patients with advanced and symptomatic DTC that cannot be treated with further surgery or RAI may benefit from systemic therapy. Older cytotoxic drugs have shown little benefit for progressive, advanced, or metastatic papillary or follicular thyroid cancer while causing significant side effects. Improved understanding of the pathogenesis of these cancers is leading to the development of new agents aimed at specific oncogenic mechanisms (e.g., RET, BRAF). Currently three tyrosine kinase inhibitors (sorafenib, lenvatinib, and cabozantinib) are approved for therapy of metastatic, RAI-resistant DTC.
Sorafenib, an oral multi-kinase inhibitor, inhibits vascular endothelial growth factor receptors (VEGFR-1, VEGFR-2, and VEGFR-3), RET kinase (including RET/PTC), BRAF V600E, and platelet-derived growth factor receptor (PDGFR) beta. In the DECISION phase 3 multicenter placebo-controlled trial of 416 patients, 409 had distant metastases: 86% in the lungs, 51% in lymph nodes, and 27% in bone (103). The group treated with sorafenib had longer progression-free survival (10.8 months) compared to the placebo group (5.8 months). At disease progression, 71% of patients in the placebo group crossed over to receive open-label sorafenib; as a consequence, overall survival did not differ between the two groups. Twenty percent of patients in the sorafenib group received other cancer therapy after the trial. The most frequent adverse events in the active drug group were palmar-plantar erythrodysesthesia, diarrhea, alopecia, rash, weight loss, hypertension, anorexia, oral mucositis and pruritus. Side effects were relieved by dose reduction.
Lenvatinib is a tyrosine kinase inhibitor of the VEGFRs 1, 2, and 3; fibroblast derived growth factor receptor (FGFR)s 1 through 4; PDGFRα; RET; and KIT signaling pathways. The SELECT phase 3 trial randomly assigned 261 patients to receive lenvatinib and 131 patients to receive placebo; the median age of patients in the trial was over 60 years (104). The median duration of follow-up was 17 months; 114 patients assigned placebo had progression, and 109 of them elected to receive lenvatinib. Disease progression occurred in 36% in the lenvatinib group compared to 83% in the placebo group. Median progression free survival was 18.3 months with lenvatinib versus 3.6 months with placebo. Disease response rate was 66% with lenvatinib compared with 1.5% with placebo. The benefit appeared in all subgroups, including all histologic types of tumor. Adverse events occurred in 97% of patients taking lenvatinib and in 60% taking placebo; the main adverse events were hypertension, diarrhea, fatigue, decreased appetite, palmar-plantar erythrodysesthesia, proteinuria, renal failure, and thromboembolic events.
Cabozantinib, another kinase inhibitor, was approved for use in metastatic, RAI-refractory DTC based upon the findings of the COSMIC-311 trial (105). This study showed longer progression free survival with cabozantinib treatment (n=125) compared to placebo (n=62): median not reached (96% CI 5.7 months-not estimable [NE]) versus 1.9 months (CI 1.8-3.6); hazard ratio 0·22 (96% CI 0.13-0.36; p<0·0001). Of note, patients included in this trial must have previously progressed on prior sorafenib and/or lenvatinib therapy, and those in the placebo arm were allowed to cross over to cabozantinib therapy on disease progression. Side effects of cabozantinib were similar to other kinase inhibitors, including palmar-plantar erythrodysesthesia, hypertension, and fatigue.
In 2020, two RET inhibitors, selpercatinib and pralsetinib, were approved for the treatment of advanced or metastatic RET-altered thyroid cancers, including medullary and follicular cell-derived. Selpercatinib (LIBRETTO-001) showed an overall response rate of 79% (95% CI, 54 to 94), and 1-year progression-free survival of 64% (95% CI, 37 to 82) in 19 patients with previously treated RET fusion-positive thyroid cancer (inclusive of PTC, Hurthle cell, poorly differentiated and analplastic) (106). The most common high grade (3-4) adverse events were hypertension, hepatotoxicity, hyponatremia, and diarrhea (106). A recently published update of LIBRETTO-001 outcomes (107), showed a persistent overall response rate of 77.3% (95% CI 54.6-92.2) with a median duratrion of response of 18.4 months (95% CI 10.1-not reached) and 68.6% (95% CI 42.8-84.6) of patients showing continued progression free survival >12 months. The efficacy evaluation for pralsetinib in patients with RET fusion–positive thyroid cancer was based on the phase I/II ARROW study (108) evaluating nine patients with RAI-refractory PTC; 56% had received prior lenvatinib and/or sorafenib and 22% had received cabozantinib and/or vandetanib. The overall response rate was 89% (95% CI 52-100), with all eight patients demonstrating partial response to treatment and effects lasting >6 months. Notable adverse events seen in thyroid cancer patients included hypertension, fatigue, cytopenia, and pneumonitis (108). Finally, larotrectinib and entrectinib are approved for metastatic or unresectable thyroid cancers with NTRK gene fusions and no alternative treatments (109, 110).
While not without side effects, these targeted kinase and RET inhibitors demonstrated efficacy in prolonging disease free survival in patients with metastatic, RAI-refractory DTC and should be considered in the symptomatic elderly patient with sufficient performance status and potential benefit. For differentiated thyroid cancer that progresses despite these therapies, additional treatment with external beam radiation, off label use of BRAF inhibitors, and clinical trials of immune checkpoint inhibitor therapies are sometimes utilized. These modalities are discussed below in the context of anaplastic thyroid cancer.
REDIFFERENTIATION THERAPY
Another treatment approach that has been explored in advanced, RAI-resistant follicular cell-derived thyroid cancers is the use of MEK or BRAF inhibitors for redifferentiation and restoration of RAI sensitivity. Several trials have been pursued based upon promising preclinical evidence showing increased sodium-iodine symporter expression and radioiodine uptake reinduction by modulation of the MAPK signaling pathway (111). An early phase study by Ho et al. (112) showed that in 20 patients with RAI-refractory advanced DTC, treatment with MEK inhibitor selumetinib for 4 weeks increased sensitivity to RAI uptake in 12 (60%) subjects (including 4/9 with BRAF and 5/5 with NRASmutations). Eight of 12 patients reached the pre-defined dosimetry threshold for repeat RAI therapy, of which five had an objective response and three had stable disease (112).
The subsequent phase III ASTRA study (113) was done to evaluate whether selumetinib given with initial RAI therapy in DTC patients with a high risk of primary treatment failure (i.e. ATA high risk of recurrence) would improve complete remission at 18 months and decrease the need for additional therapy. The addition of selumetinib to radioiodine did not improve the complete remission rate (40% vs. 38.5% in the placebo group). Another multi-center phase II prospective trial evaluating selumetinib in RAI-refractory DTC patients is currently underway (SEL-I-METRY, trial ISRCTN17468602) (114).
Several small studies have examined other BRAF (e.g. drabafenib) and MEK inhibitors (e.g. trametinib), alone or in combination, as reviewed recently by Buffet et al. (114). For example, Rothenberg et al. (115) showed resensitization in six of 10 BRAFV600E+ RAI-resistant thyroid cancer patients following six weeks of drabafenib therapy. In addition, following RAI therapy with 150mCi, two of the six patients showed a partial response and the remaining four had stable disease at three months. Another group, Dunn et al. (116) also evaluated vemurafenib in 10 patients with a BRAF-mutated-PTC or poorly differentiated thyroid cancer. After 4 weeks of vemurafenib, RAI uptake increased in 6/10 patients, and of four patients retreated with RAI therapy, two showed partial response and two had stable disease at six months. While a potentially promising adjuvant strategy for these challenging tumors, additional prospective evaluation is needed before this strategy can be considered within the standard of care.
ANAPLASTIC THYROID CANCER
Anaplastic thyroid carcinoma (ATC) is a rare and aggressive subtype of thyroid cancer that accounts for <1% of all thyroid cancers (27, 31). It more commonly affects the elderly, with a mean age at diagnosis of 65 years and more than 90% patients with ATC are over age 50 (31). Despite recent advances, the median overall survival remains poor, around 3–5 months, with a 1-year survival of approximately 20% (117). Aldinger et al. reported a five-year survival rate of only 7.1% with a mean survival period of 6.2 months from the time of tissue diagnosis and 11.8 months from the time of onset of symptoms (118).
The most frequent presenting complaint in patients with ATC is a rapidly growing mass with tightness in the neck (118). Patients may also complain of dysphagia, hoarseness, dyspnea, neck pain, sore throat, and cough. Examination of the neck usually reveals a fixed, large, firm mass, which may impair the ability to detect lymphadenopathy on clinical examination. Hemorrhage and necrosis within the tumor may result in soft, fluctuant masses. Rarely, patients with massive tumor extension into the mediastinum or lungs may present with superior vena cava syndrome or dyspnea.
Unfortunately, most patients with ATC present with advanced stage disease. In a retrospective study of thyroid cancers in 204 elderly (age >60 years) patients by Lin et al. (47), 75% of patients diagnosed with ATC had distant metastases to the lung, bone, mediastinum, and peritoneum at presentation. Similarly, in the cohort reported by Aldinger et al., 78 of 84 (93%) patients with ATC presented with advanced stage III and stage IV disease (118). Additional patient factors associated with worse prognosis in ATC include advanced age (>60–70 years), male gender, presence of leukocytosis (>10,000), and symptoms related to tumor mass effect, such as neck pain, dysphagia, rapidly growing neck mass. Regarding older age as a poor prognostic factor, in a cohort of 516 patients with ATC, Kebebew et al. reported a 28% greater mortality in patients over 60 years of age compared to those less than 60 years determined by multivariate analysis (117).
ATC often, but not always, arises from pre-existing differentiated thyroid cancer, with 20% of patients with antecedent DTC and another 20-30% with concurrent DTC (co-existent on histopathology). There is also a higher incidence of ATC in patients with endemic goiter. These associations are relevant for the treatment of ATC because driver mutations such as BRAF and RAS may be retained in the anaplastic tumor cells and can be targeted with therapy (31, 118).
Treatment of Anaplastic Thyroid Cancer
While the prognosis of ATC remains poor, treatment options to slow the progression of disease, palliate symptoms, and, in rare cases, attempt cure, are available as approved therapies and in clinical trials.
EXTERNAL RADIATION
External radiation to the neck region is appropriate for patients with aggressive cancers that cannot be completely resected surgically (12). Schwartz et al. reported limited success in the treatment of RAI-refractory patients with extrathyroidal spread, positive surgical margins, or gross residual disease with a mean of 60 Gy (38-72 Gy); survival was less in patients with high-risk pathology, metastases, and gross residual disease (119). In the context of ATC, disease is often assumed to be radioiodine refractory, and external beam radiation may be used for preservation of vital neck structures.
TARGETED SYSTEMIC THERAPY AND IMMUNOTHERAPY
Most patients with ATC have rapidly progressive disease and should be evaluated for clinical trials when feasible as new treatments continue to be developed. Targeted therapy with inhibitors to specific gene mutations and fusions has shown some success and is the focus of numerous ongoing clinical trials. Therapies include inhibitors of BRAF, MEK, NTRK, RET, and ALK. Combination treatment with BRAF inhibitor dabrafenib and MEK inhibitor trametinib was recently approved for the treatment of BRAFV600E mutated, unresectable/locally advanced ATC, following a 69% overall response rate in a phase II open label trial of 16 patients with ATC (120).
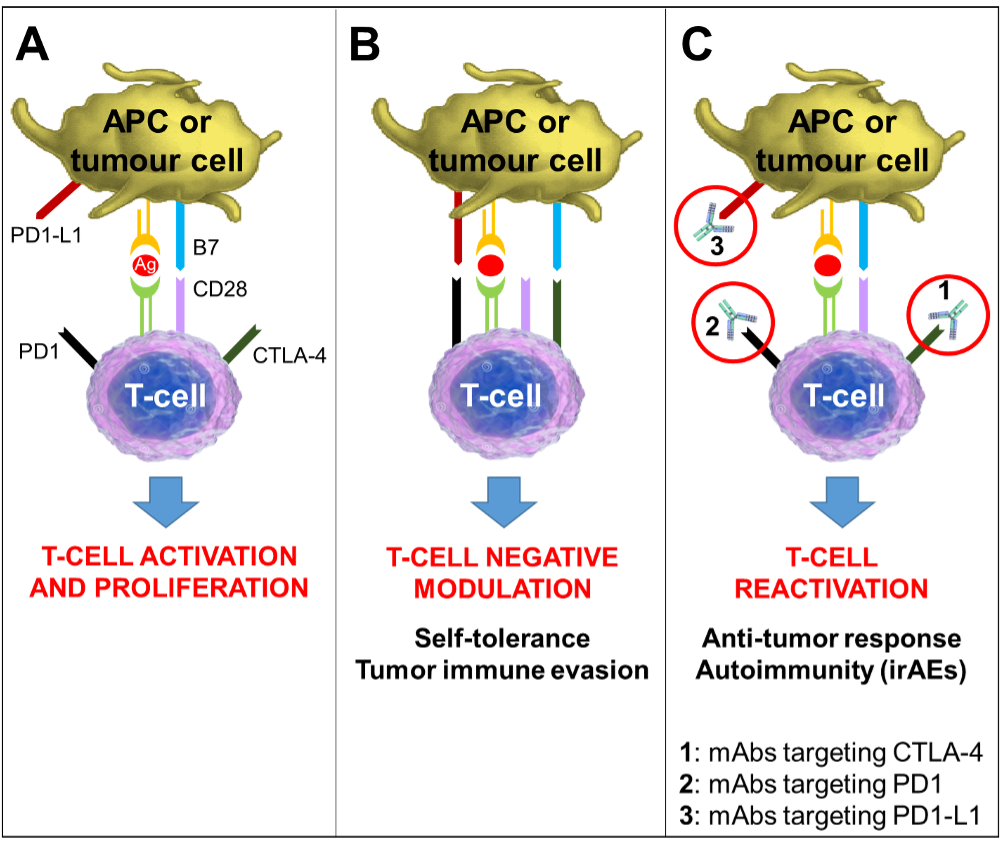
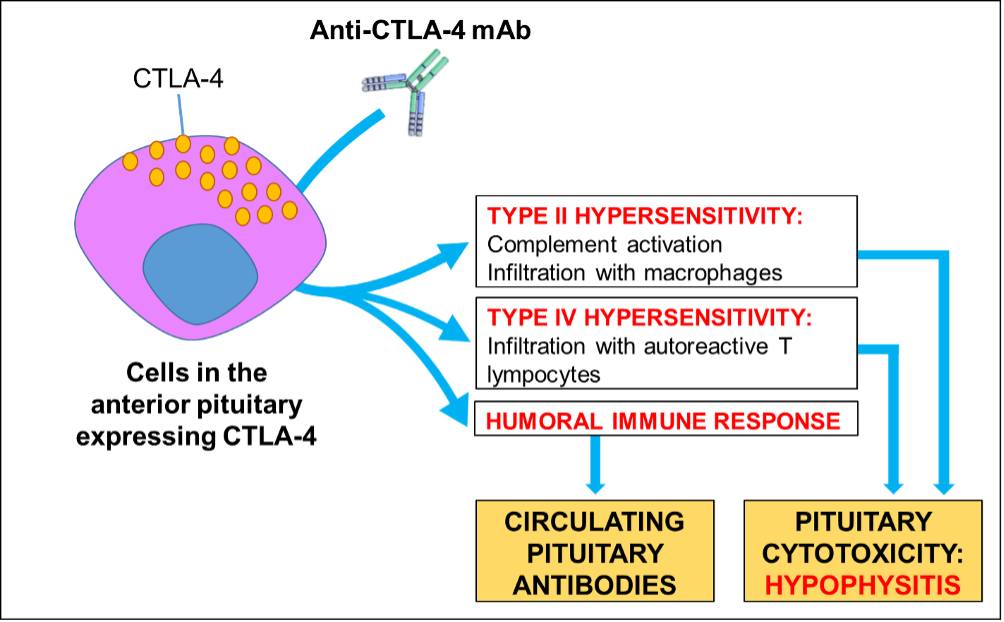
Immunotherapy reagents target the impaired immune responses and immune suppression that arise in cancer allowing malignant cells to grow and spread. Immune checkpoint inhibitors are a kind of immunotherapy that block immune regulatory pathways with the goal of increasing anti-tumor immune responses and producing tumor killing by host leukocytes. Two primary classes of immunotherapy being evaluated for advanced thyroid cancer are inhibitors of cytotoxic T lymphocyte A (CTLA)-4 (such as ipilimumab) and inhibitors of programmed cell death (PD) receptor/ligand interactions (nivolumab, pembrolizumab, atezolizumab). Currently, immune checkpoint inhibitors are being evaluated alone and in combination with targeted therapies for ATC (120).
MEDULLARY THYROID CANCER
Medullary thyroid cancer (MTC) constitutes approximately 2-5% of all thyroid malignancies, but it is responsible for up to 13.4% of all deaths from thyroid cancer (30, 121). It is a well-differentiated type of tumor that arises from the parafollicular C cells of the thyroid gland, and therefore it is categorized as a neuroendocrine tumor. In 80% of patients, medullary thyroid cancer occurs sporadically, but in about 20% of patients there is a family history of medullary carcinoma. Familial MTC is inherited in an autosomal dominant pattern with nearly complete penetrance. A germline mutation in the RET proto-oncogene, which encodes a transmembrane tyrosine kinase receptor, predisposes individuals to develop hereditary MTC. In the sporadic form, the tumor occurs as a result of a mutation involving only the somatic cells. Sporadic forms of MTC are more common in older patients (mean age at presentation 47 years), while the hereditary forms of MTC are more common in younger patients (121). The prevalence of MTC is nearly equal in males and females.
Parafollicular cells secrete calcitonin, and in MTC this protein is greatly elevated and serum level correlates directly with the burden of disease (122). Other neuroendocrine cell products, including histamine, serotonin, prolactin, vasoactive intestinal polypeptide, and prostaglandin, can be elevated in patients with MTC and lead to systemic symptoms such as diarrhea or flushing (122). In some cases, Cushing’s syndrome may develop as a result of ectopic adrenocorticotrophic hormone (ACTH) secretion from the tumor. The typical presentation of MTC is a palpable nodule in the upper part of the thyroid lobe, and the presence of systemic symptoms is almost universally associated with distant metastases (37). In the retrospective report of 104 patients with MTC by Kebebew et al., 74% of the patients in the sporadic group presented with a thyroid mass, 16% had local symptoms (dysphagia, dyspnea, or hoarseness), and 10% had systemic symptoms (bone pain, flushing, and/or diarrhea) attributable to the cancer (121).
Within MTC, older age at diagnosis has been associated with a worse prognosis. Kebebew et al. followed patients with MTC for a mean time of 8.6 years and found that advanced age and stage at diagnosis were independent predictors of worse survival (121). The 5-year survival rates by stage were 100% (stage I), 90% (stage II), 86% (stage III), and 55% (stage IV). The highest survival was seen in female patients under age 45 with MTC confined to the thyroid (121). Saad et al. similarly reported that patients younger than 40 years old at diagnosis had a significantly better survival rate in MTC (122). Scopsi et al. reported a worse prognosis in patients with sporadic MTC who had extrathyroidal tumor invasion, distant metastases, or age greater than 60 years at the time of diagnosis (123). Interestingly, a more recent study that adjusted for baseline age-related mortality in the general population found no significant association with age and prognosis in MTC (124). This raises similar questions to those posed recently for differentiated thyroid cancer as to whether age truly has an independent role in prognosis for these thyroid cancers apart from the general increase in morbidity and mortality with aging.
Treatment of Medullary Thyroid Cancer
The standard treatment for MTC is surgical resection (total thyroidectomy) with regional lymph node dissection, with routine bilateral central neck dissection and consideration of lateral neck dissection in patients with large primary tumors (>1 cm) or pre-operative imaging with involved nodes. Successful complete surgical resection is associated with improved prognosis. In patients with disease restricted to the thyroid gland and without nodal involvement, the risk of recurrence and mortality is very low, compared to those with nodal disease at presentation (125).
Serum calcitonin and CEA levels are trended post-operatively to monitor for residual or recurrent disease, beginning around 2-3 months after surgery. A rise in either tumor marker should prompt imaging to look for recurrent disease. Radioactive iodine is not indicated in the treatment of MTC as parafollicular cells do not express NIS or concentrate iodine. Additionally, thyroid hormone replacement is required following thyroidectomy, with TSH targeted to the normal range rather than suppression (126). TSH does not stimulate the growth of parafollicular cells.
In patients with progressive or metastatic disease not amenable to surgery, tyrosine kinase inhibitors vandetanib and cabozantinib may be used. Vandetanib is an oral inhibitor that targets VEGFR, RET, and epidermal growth factor receptor (EGFR). In the international, randomized controlled phase III ZETA trial of vandetanib 300 mg per day that included over 300 patients with unresectable, locally advanced or metastatic sporadic or hereditary MTC, progression-free survival was significantly greater for patients treated with vandetanib (hazard ratio 0.46, 95% CI 0.31-0.69 versus placebo) (127). Adverse events occurred more commonly with vandetanib compared to placebo, including diarrhea, nausea, palmar-plantar erythrodysesthesia, hypertension, and headache.
Cabozantinib (128) is another oral tyrosine kinase inhibitor targeting MET, VEGFR2, and RET signaling pathways. The phase III international, randomized controlled EXAM trial evaluated cabozantinib versus placebo in the treatment of 330 patients with progressive, metastatic MTC, with a primary outcome of progression free survival (PFS). Median PFS was 11.2 months for cabozantinib versus 4.0 months for placebo (hazard ratio, 0.28; 95% CI, 0.19 to 0.40; P <0.001), with benefit seen across all subgroups including age, prior TKI treatment, and RET mutation status (hereditary or sporadic). Response rate was 28% for cabozantinib and 0% for placebo. Common cabozantinib-associated adverse events noted in the trial included diarrhea, palmar-plantar erythrodysesthesia, decreased weight, nausea, and fatigue.
As discussed above, two targeted RET-fusion inhibitors have recently been approved for use in MTC: selpercatinib (106) and pralsetinib (108). Selpercatinib showed a 69% (95% CI 55-81) overall response rate and 82% (95% CI 69-90) one year progression free survival in 55 patients with RET-mutated MTC patients who previously had failed treatment with vandetanib, cabozantinib, or both (106). In addition, in 88 patients RET-mutated MTC but without prior systemic therapy, the study found a 73% (95% CI 62-82) overall response and 92% (95% CI 82-97) one year progression free survival (106). Similarly, in the phase I/II study of pralsetinib, the overall response rates were 60% (95% CI 47-63) for patients with RET-mutated MTC patients who had previously received cabozantinib or vandetanib, or both, and 71% (95% CI 48–89) in patients with treatment-naive RET-mutated MTC (108). Side effects for both RET inhibitors were similar to those seen in follicular cell-derived thyroid cancer patients discussed above.
Given the poor prognosis of MTC, continued development of new treatment strategies is needed and management at a center experienced with this type of cancer is recommended.
CONCLUSION
In summary, thyroid nodules and cancer are common in elderly patients and demonstrate age-specific prevalence, malignancy risk, and clinical behavior. Co-morbid conditions and patient preference should inform management of these entities in the elderly, with particular attention to the risks of surgery and medication adverse effects. More research is needed to understand the mechanisms underlying the distinct clinical behavior of thyroid cancer found in older patients, including the drivers of more advanced stage at presentation, higher recurrence risk, and greater mortality.
REFERENCES
- Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15- year study of the incidence of thyroid malignancy. Ann Intern Med. 1968;69:537–540. [PubMed: 5673172]
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf). 1977;7:481–493. [PubMed: 598014]
- Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231. [PubMed: 9027275]
- Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706. [PubMed: 19601965]
- Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:901–911. [PubMed: 19041821]
- Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, Krane JF, Barletta JA, Kim MI, Larsen PR, Alexander EK. The Influence of Patient Age on Thyroid Nodule Formation, Multinodularity, and Thyroid Cancer Risk. J Clin Endocrinol Metab. 2015 Dec;100(12):4434–40. [PubMed: 26465395]
- Mazzaferri E. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559. [PubMed: 8426623]
- Ezzat S, Sarti DA, Cain DR, et al. Thyroid incidentalomas prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840. [PubMed: 8053752]
- Dauksiene D, Petkeviciene J, Klumbiene J, Verkauskiene R, Vainikonyte-Kristapone J, Seibokaite A, Ceponis J, Sidlauskas V, Daugintyte-Petrusiene L, Norkus A, Zilaitiene B. Factors Associated with the Prevalence of Thyroid Nodules and Goiter in Middle-Aged Euthyroid Subjects. Int J Endocrinol. 2017;2017:8401518. [PubMed: 28356911]
- Diez JL. Goiter in adult patients aged 55 years and older: etiology and clinical features in 634 patients. J Gerontol A Biol Sci Med Sci. 2005;60:920–923. [PubMed: 16079218]
- Schlumberger M, Filetti S, Hay I. Nontoxic Diffuse and Nodular Goiter and Thyroid Neoplasia. In: Melmed S, Williams RH, editors. Williams Textbook of Endocrinology. 12th ed. Philadelphia: Elsevier/Saunders; 2011. p 440-449.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016 Jan;26(1):1–133. [PubMed: 26462967]
- Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, Hammers LW, Hamper UM, Langer JE, Reading CC, Scoutt LM, Stavros AT. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587–595. [PubMed: 28372962]
- Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J. 2017;6(5):225–237. [PubMed: 29167761]
- Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, Lim HK, Moon WJ, Na DG, Park JS, Choi YJ, Hahn SY, Jeon SJ, Jung SL, Kim DW, Kim EK, Kwak JY, Lee CY, Lee HJ, Lee JH, Lee JH, Lee KH, Park SW, Sung JY, Korean Society of Thyroid R, Korean Society of R. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol. 2016;17(3):370–395. [PubMed: 27134526]
- Kim PH, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Unnecessary thyroid nodule biopsy rates under four ultrasound risk stratification systems: a systematic review and meta-analysis. Eur Radiol. 2021 May;31(5):2877-2885. [PubMed: 33057762]
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2017 Nov;27(11):1341–1346. [PubMed: 29091573]
- Livhits MJ, Zhu CY, Kuo EJ, Nguyen DT, Kim J, Tseng CH, Leung AM, Rao J, Levin M, Douek ML, Beckett KR, Cheung DS, Gofnung YA, Smooke-Praw S, Yeh MW. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol. 2021 Jan 1;7(1):70-77. [PubMed: 33300952]
- Nikiforov YE, Carty SE, Chiosea SI, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120:3627–34. [PubMed: 25209362]
- Nikiforova MN, Mercurio S, Wald AI, Barbi de Moura M, Callenberg K, Santana-Santos L, Gooding WE, Yip L, Ferris RL, Nikiforov YE. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018 Apr 15;124(8):1682–1690. [PubMed: 29345728]
- Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–15. [PubMed: 22731672]
- Patel KN, Angell TE, Babiarz J, Barth NM, Blevins T, Duh QY, Ghossein RA, Harrell RM, Huang J, Kennedy GC, Kim SY, Kloos RT. LiVolsi VA, Randolph GW, Sadow PM, Shanik MH, Sosa JA, Traweek ST, Walsh PS, Whitney D, Yeh MW, Ladenson PW. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg. 2018 Sep 1;153(9):817–824. [PubMed: 29799911]
- Labourier E, Shifrin A, Busseniers AE, Lupo MA, Manganelli ML, Andruss B, Wylie D, Beaudenon-Huibregtse S. Molecular Testing for miRNA, mRNA, and DNA on Fine-Needle Aspiration Improves the Preoperative Diagnosis of Thyroid Nodules With Indeterminate Cytology. J Clin Endocrinol Metab. 2015 Jul;100(7):2743–50. [PubMed: 25965083]
- Wang Z, Vyas CM, Van Benschoten O, Nehs MA, Moore FD Jr, Marqusee E, Krane JF, Kim MI, Heller HT, Gawande AA, Frates MC, Doubilet PM, Doherty GM, Cho NL, Cibas ES, Benson CB, Barletta JA, Zavacki AM, Larsen PR, Alexander EK, Angell TE. Quantitative Analysis of the Benefits and Risk of Thyroid Nodule Evaluation in Patients ≥70 Years Old. Thyroid. 2018 Apr;28(4):465–471. [PubMed: 29608439]
- Smith SA, Hay ID, Goellner JR, et al. Mortality from papillary thyroid carcinoma: a case-control study of 56 lethal cases. Cancer. 1988;62:1381–1388. [PubMed: 3416277]
- Tollefsen HR, DeCosse JJ, Hutter RVP. Papillary carcinoma of the thyroid: a clinical and pathological study of 70 fatal cases. Cancer. 1964;17:1035–1044. [PubMed: 14202591]
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
- Girardi FM. Thyroid Carcinoma Pattern Presentation According to Age. Int Arch Otorhinolaryngol. 2017 Jan;21(1):38–41. [PubMed: 28050206]
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. [PubMed: 16684987]
- Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. 2017 Apr 4;317(13):1338–1348. [PubMed: 28362912]
- Lebastchi AH, Callender GG. Thyroid cancer. Curr Probl Cancer. 2014 Mar-Apr;38(2):48–74. [PubMed: 24951026]
- Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016 Dec 3;388(10061):2783–2795. [PubMed: 27240885]
- Gupta KL. Neoplasm of the thyroid gland. Clin Geriatr Med. 1995;11:271–290. [PubMed: 7606696]
- Sarne D, Schneider AB. External radiation and thyroid neoplasia. Endocrinol Metab Clin North Am. 1996;25(1):181–195. [PubMed: 8907686]
- Ron E, Kleinerman RA, Boice JD Jr. LiVolsi VA, Flannery JT, Fraumeni JF Jr. A population-based case-control study of thyroid cancer. J Natl Cancer Inst. 1987;79(1):1–12. [PubMed: 3474436]
- Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. 1995. Radiat Res. 2012;178(2):AV43–AV60. [PubMed: 22870979]
- Lee YS , Lim H , Chang HS , Park CS. Papillary thyroid microcarcinomas are different from latent papillary thyroid carcinomas at autopsy. J Korean Med Sci. 2014;29:676–679. [PubMed: 24851024]
- Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27–34. [PubMed: 24001104]
- Grebe SK, Hay ID. Follicular thyroid cancer. Endocrinol Metab Clin North Am. 1995;24:761. [PubMed: 8608779]
- Brennan MD, Bergstralh EJ, Van Heerden JA, et al. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66:11–22. [PubMed: 1988751]
- Grani G, Lamartina L, Durante C, Filetti S, Cooper DS. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018 Jun;6(6):500–514. [PubMed: 29102432]
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013 Mar;13(3):184–99. [PubMed: 23429735]
- Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998 Dec 15;83(12):2638–48. [PubMed: 9874472]
- Máximo V, Lima J, Prazeres H, Soares P, Sobrinho-Simões M. The biology and the genetics of Hürthle cell tumors of the thyroid. Endocr Relat Cancer. 2016 Dec;23(12):X2. [PubMed: 27807063]
- Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, Nanjangud G, Eng S, Bose P, Kuo F, Morris LGT, Landa I, Carrillo Albornoz PB, Riaz N, Nikiforov YE, Patel K, Umbricht C, Zeiger M, Kebebew E, Sherman E, Ghossein R, Fagin JA, Chan TA. Integrated Genomic Analysis of Hürthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell. 2018 Aug 13;34(2):256–270.e5. [PubMed: 30107176]
- Gopal RK, Kübler K, Calvo SE, Polak P, Livitz D, Rosebrock D, Sadow PM, Campbell B, Donovan SE, Amin S, Gigliotti BJ, Grabarek Z, Hess JM, Stewart C, Braunstein LZ, Arndt PF, Mordecai S, Shih AR, Chaves F, Zhan T, Lubitz CC, Kim J, Iafrate AJ, Wirth L, Parangi S, Leshchiner I, Daniels GH, Mootha VK, Dias-Santagata D, Getz G, McFadden DG. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hürthle Cell Carcinoma. Cancer Cell. 2018 Aug 13;34(2):242–255.e5. [PubMed: 30107175]
- Lin JD, Chao TC, Chen ST, et al. Characteristics of thyroid carcinomas in aging patients. Eur J Clin Invest. 2000;30:147–153. [PubMed: 10651840]
- Chereau N, Trésallet C, Noullet S, Godiris-Petit G, Tissier F, Leenhardt L, Menegaux F. Prognosis of papillary thyroid carcinoma in elderly patients after thyroid resection: A retrospective cohort analysis. Medicine (Baltimore). 2016 Nov;95(47):e5450. [PubMed: 27893690]
- Lerch H, Schober O, Kuwert T, et al. Survival of differentiated thyroid carcinoma studied in 500 patients. J Clin Onc. 1997;15:2067–2075. [PubMed: 9164219]
- Shi LY, Liu J, Yu LJ, Lei YM, Leng SX, Zhang HY. Clinic-pathologic Features and Prognostic Analysis of Thyroid Cancer in the Older Adult: A SEER Based Study. J Cancer. 2018 Jul 1;9(15):2744–2750. [PubMed: 30087716]
- Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist. 2009 Mar;14(3):216–21. [PubMed: 19270027]
- Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, Hyslop T, Roman SA, Sosa JA. Presence and Number of Lymph Node Metastases Are Associated With Compromised Survival for Patients Younger Than Age 45 Years With Papillary Thyroid Cancer. J Clin Oncol. 2015 Jul 20;33(21):2370–5. [PubMed: 26077238]
- Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid. 2015 Jan;25(1):125–32. [PubMed: 25369076]
- Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha A, Tuttle RM, Shah JP, Patel SG. Survival from Differentiated Thyroid Cancer: What Has Age Got to Do with It? Thyroid. 2015 Oct;25(10):1106–14. [PubMed: 26148759]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Weinheim, Germany, 2017; pp. 69–71.
- Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993 Dec;114(6):1050–7. [PubMed: 8256208]
- Davis NL, Bugis SP, McGregor GI, Germann E. An evaluation of prognostic scoring systems in patients with follicular thyroid cancer. Am J Surg. 1995 Nov;170(5):476–80. [PubMed: 7485736]
- Halnan KE. Influence of age and sex on incidence and prognosis of thyroid cancer. Cancer. 1966;19:1534–1536. [PubMed: 5925260]
- Cady B, Sedgwick CE, Meissner WA, et al. Risk factor analysis in differentiated thyroid cancer. Cancer. 1979;43:810–820. [PubMed: 427722]
- Ito Y, Miyauchi A, Jikuzono T, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Ichihara K, Kuma K. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg. 2007 Apr;31(4):838–48. [PubMed: 17347900]
- Sugino K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Yano Y, Uruno T, Akaishi J, Kameyama K, Ito K. Prognosis and prognostic factors for distant metastases and tumor mortality in follicular thyroid carcinoma. Thyroid. 2011 Jul;21(7):751–7. [PubMed: 21615311]
- Voutilainen PE, Multanen MM, Leppaniemi AK, et al. Prognosis after lymph node recurrence in papillary thyroid carcinoma depends on age. Thyroid. 2001;11:953. [PubMed: 11716043]
- Zaydfundim V, Feurer ID, Griffin MR, et al. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144:1070–1077. [PubMed: 19041020]
- Ito Y, Kudo T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Prognostic factors of papillary thyroid carcinoma vary according to sex and patient age. World J Surg. 35:2684-2690, 2011. [PubMed: 21953129]
- Ylli D, Burman KD, Van Nostrand D, Wartofsky L. Eliminating the Age Cutoff in Staging of Differentiated Thyroid Cancer: The Safest Road? J Clin Endocrinol Metab. 2018 May 1;103(5):1813–1817. [PubMed: 29741712]
- Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D, Vaisman M, Carvalho A, Learoyd D, Leslie WD, Nason RW, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Pathak KA, Shen WT, Sywak M, Kowalski L, Freeman J, Perrier N, Shah JP. An International Multi-Institutional Validation of Age 55 Years as a Cutoff for Risk Stratification in the AJCC/UICC Staging System for Well-Differentiated Thyroid Cancer. Thyroid. 2016 Mar;26(3):373–80. [PubMed: 26914539]
- Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Shah JP. Defining a Valid Age Cutoff in Staging of Well-Differentiated Thyroid Cancer. Ann Surg Oncol. 2016 Feb;23(2):410–5. [PubMed: 26215199]
- Lechner MG, Bernardo AC, Lampe A, Praw SS, Tam SH, Angell TE. Changes in Stage Distribution and Disease-Specific Survival in Differentiated Thyroid Cancer with Transition to American Joint Committee on Cancer 8th Edition: A Systematic Review and Meta-Analysis. Oncologist. 2021 Feb;26(2):e251-e260. [PubMed: 32864832]
- Papaleontiou M, Norton EC, Reyes-Gastelum D, Banerjee M, Haymart MR. Competing Causes of Death in Older Adults with Thyroid Cancer. Thyroid. 2021 Sep;31(9):1359-1365. [PubMed: 33764188]
- McLeod DS, Carruthers K, Kevat DA. Optimal differentiated thyroid cancer management in the elderly. Drugs Aging. 2015 Apr;32(4):283–94. [PubMed: 25825123]
- Randolph GW, Doherty GM. Carcinoma of the follicular thyroid: surgical therapy. In: Braverman LE, Cooper DS, editors. Werner & Ingbar’s the thyroid: a fundamental and clinical text. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p. 702–11.
- Bliss R, Patel N, Guinea A, et al. Age is no contraindication to thyroid surgery. Age Ageing. 1999;28:363–6. [PubMed: 10459789]
- Passler C, Avanessian R, Kaczirek K, et al. Thyroid surgery in the geriatric patient. Arch Surg. 2002;137:1243–8. [PubMed: 12413310]
- Seybt MW, Khichi S, Terris DJ. Geriatric thyroidectomy: safety of thyroid surgery in an aging population. Arch Otolaryngol Head Neck Surg. 2009;135:1041–4. [PubMed: 19841346]
- Raffaelli M, Bellantone R, Princi P, et al. Surgical treatment of thyroid diseases in elderly patients. Am J Surg. 2010;200:467–72. [PubMed: 20887839]
- Sosa JA, Mehta PJ, Wang TS, et al. A population-based study of outcomes from thyroidectomy in aging Americans: at what cost? J Am Coll Surg. 2008;206:1097–105. [PubMed: 18501806]
- Tuggle CT, Park LS, Roman S, et al. Rehospitalization among elderly patients with thyroid cancer after thyroidectomy are prevalent and costly. Ann Surg Oncol. 2010;17:2816–23. [PubMed: 20552406]
- Kandil E, Krishnan B, Noureldine SI, Yao L, Tufano RP. Hemithyroidectomy: a meta-analysis of postoperative need for hormone replacement and complications. ORL J Otorhinolaryngol Relat Spec. 2013;75(1):6-17. [Pubmed: 23486083].
- Schumm MA, Lechner MG, Shu ML, Ochoa JE, Kim J, Tseng CH, Leung AM, Yeh MW. Frequency of Thyroid Hormone Replacement After Lobectomy for Differentiated Thyroid Cancer. Endocr Pract. 2021 Jul;27(7):691-697. [PubMed: 33642257]
- Tuttle RM, Zhang L, Shaha A. A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Expert Rev Endocrinol Metab. 2018 Mar;13(2):77-85. [PubMed: 30058863]
- Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014 Mar;38(3):673-8. [Pubmed: 24233662].
- Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, Untch B, Ganly I, Shaha AR, Shah JP, Pace M, Li D, Bach A, Lin O, Whiting A, Ghossein R, Landa I, Sabra M, Boucai L, Fish S, Morris LGT. Natural History and Tumor Volume Kinetics of Papillary Thyroid Cancers During Active Surveillance. JAMA Otolaryngol Head Neck Surg. 2017 Oct 1;143(10):1015-1020. [Pubmed: 28859191]
- Tufano RP, Shindo M, Shaha AR. New Recommendations for Extent of Thyroidectomy and Active Surveillance for the Treatment of Differentiated Thyroid Cancer. JAMA Otolaryngol Head Neck Surg. 2016 Jul 1;142(7):625-6. [PubMed: 27196622].
- Ruel E , Thomas S , Dinan M , Perkins JM , Roman SA , Sosa JA. Adjuvant radioactive iodine therapy is associated with improved survival for patients with intermediate-risk papillary thyroid cancer. J Clin Endocrinol Metab. 2015;100:1529–1536. [PubMed: 25642591]
- Schlumberger M, Catargi B, Borget I, et al. .Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673 [PubMed: 22551127]
- Mallick U, Harmer C, Yap B, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–1685. [PubMed: 22551128]
- Schlumberger M, Leboulleux S, Catargi B, et al. Outcome after ablation in patients with low-risk thyroid cancer (ESTIMABL1): 5-year follow-up results of a randomised, phase 3, equivalence trial. Lancet Diabetes Endocrinol. 2018;6:618–626. [PubMed: 29807824]
- Andresen NS, Buatti JM, Tewfik HH, Pagedar NA, Anderson CM, Watkins JM. Radioiodine Ablation following Thyroidectomy for Differentiated Thyroid Cancer: Literature Review of Utility, Dose, and Toxicity. Eur Thyroid J. 2017 Jul;6(4):187–196. [PubMed: 28868259]
- Flynn RW, Bonellie SR, Jung RT, et al. Serum thyroid stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;95:186–93. [PubMed: 19906785]
- Bauer DC, Ettinger B, Nevitt MC, et al. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med. 2001;134:561–8. [PubMed: 12803168]
- Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab. 1996;81:1232–6. [PubMed: 8772604]
- Sugitani I, Fujimoto Y. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: a prospective controlled study. Surgery. 2011;150:1250–7. [PubMed: 22136848]
- Wang LY, Smith AW, Palmer FL, Tuttle RM, Mahrous A, Nixon IJ, Patel SG, Ganly I, Fagin JA, Boucai L. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid. 2015 Mar;25(3):300–7. [PubMed: 25386760]
- Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R., National Osteoporosis Foundation. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014 Oct;25(10):2359–81. [PubMed: 25182228]
- Gregerman RI, Gaffney GW, Shock NW, et al. Thyroxine turnover in euthyroid man with special reference to changes with age. J Clin Invest. 1962;41:2065–74. [PubMed: 13950233]
- Rosenbaum RL, Barzel US. Levothyroxine replacement dose for primary hypothyroidism decreases with age. Ann Intern Med. 1982;96:53–5. [PubMed: 7053703]
- Singh N, Weisler SL, Hershman JM. The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid. 2001 Oct;11(10):967–71. [PubMed: 11716045]
- Kim M, Ladenson P. Thyroid. In: Goldman L, Schafer AI, editors. Goldman’s Cecil medicine. 24th ed. Philadelphia: Elsevier Saunders; 2012. p 1450–63.
- Soroushyari A, Do D, Langton J, et al. Partial withdrawal of levothyroxine to stimulate serum thyroglobulin for thyroid cancer monitoring. Thyroid. 2004;14:1105–1107. [PubMed: 15666427]
- Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods - Strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2013 Oct;27(5):701–12. [PubMed: 24094640]
- Pedrazzini L, Baroli A, Lomuscio G, Marzoli L. Prevalence, clinical significance and prognostic value of anti-thyroglobulin antibodies in the follow-up of patients with differentiated thyroid carcinoma: a retrospective study. Minerva Endocrinol. 2009 Sep;34(3):195-203. [PubMed: 19859043].
- Momesso DP, Vaisman F, Yang SP, Bulzico DA, Corbo R, Vaisman M, Tuttle RM. Dynamic Risk Stratification in Patients with Differentiated Thyroid Cancer Treated Without Radioactive Iodine. J Clin Endocrinol Metab. 2016 Jul;101(7):2692-700. [Pubmed: 27023446]
- Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. [PubMed: 24768112]
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 372:621-30, 2015. [PubMed: 25671254]
- Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, Hoff AO, Hitre E, Bowles DW, Hernando J, Faoro L, Banerjee K, Oliver JW, Keam B, Capdevila J. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021 Aug;22(8):1126-1138. [PubMed: 34237250].
- Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux S, Godbert Y, Barlesi F, Morris JC, Owonikoko TK, Tan DSW, Gautschi O, Weiss J, de la Fouchardière C, Burkard ME, Laskin J, Taylor MH, Kroiss M, Medioni J, Goldman JW, Bauer TM, Levy B, Zhu VW, Lakhani N, Moreno V, Ebata K, Nguyen M, Heirich D, Zhu EY, Huang X, Yang L, Kherani J, Rothenberg SM, Drilon A, Subbiah V, Shah MH, Cabanillas ME. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med. 2020 Aug 27;383(9):825-835. doi: 10.1056/NEJMoa2005651. [PubMed: 32846061]
- Sherman EJ, Wirth LJ, Shah MH, et al. Selpercatinib efficacy and safety in patients with RET-altered thyroid cancer: A clinical trial update. Journal of Clinical Oncology 2021 39:15_suppl, 6073-6073
- Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, Brose MS, Zhu VW, Leboulleux S, Bowles DW, Baik CS, Adkins D, Keam B, Matos I, Garralda E, Gainor JF, Lopes G, Lin CC, Godbert Y, Sarker D, Miller SG, Clifford C, Zhang H, Turner CD, Taylor MH. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021 Aug;9(8):491-501. doi: 10.1016/S2213-8587(21)00120-0. [PubMed: 34118198].
- Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. . Larotrectinib in Patients With TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol (2020) 21(4):531–40. 10.1016/S1470-2045(19)30856-3;
- Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. . Entrectinib in Patients With Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol (2020) 21(2):271–82. 10.1016/S1470-2045(19)30691-6)
- Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, Bollag G, Kolesnick R, Thin TH, Rosen N, Zanzonico P, Larson SM, Refetoff S, Ghossein R, Fagin JA. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011 Dec;121(12):4700-11. [PubMed: 22105174]
- Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, Pentlow KS, Zanzonico PB, Haque S, Gavane S, Ghossein RA, Ricarte-Filho JC, Domínguez JM, Shen R, Tuttle RM, Larson SM, Fagin JA. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013 Feb 14;368(7):623-32. doi: 10.1056/NEJMoa1209288. [PubMed: 23406027]
- Ho A, Dedecjus M, Wirth LJ, et al. ASTRA: a phase III, randomized, placebo-controlled study evaluating complete remission rate (CRR) with short-course selumetinib plus adjuvant radioactive iodine (RAI) in patients (pts) with differentiated thyroid cancer (DTC). Presented at: the 88 Annual Meeting of the American Thyroid Association; Washington, D.C.: October 3-7, 2018. Abstract short call oral 8.
- Buffet C, Wassermann J, Hecht F, Leenhardt L, Dupuy C, Groussin L, Lussey-Lepoutre C. Redifferentiation of radioiodine-refractory thyroid cancers. Endocr Relat Cancer. 2020 May;27(5):R113-R132. [Pubmed: 32191916].
- Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015 Mar 1;21(5):1028-35. [PubMed: 25549723].
- Dunn LA, Sherman EJ, Baxi SS, Tchekmedyian V, Grewal RK, Larson SM, Pentlow KS, Haque S, Tuttle RM, Sabra MM, Fish S, Boucai L, Walters J, Ghossein RA, Seshan VE, Ni A, Li D, Knauf JA, Pfister DG, Fagin JA, Ho AL. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers. J Clin Endocrinol Metab. 2019 May 1;104(5):1417-1428. [PubMed: 30256977]
- Kebebew E, Greenspan FS, Clark OH, et al. Anaplastic thyroid carcinoma treatment outcome and prognostic factors. Cancer. 2005;103:1330–1335. [PubMed: 15739211]
- Aldinger KA, Samaan NA, Ibanez M, et al. Anaplastic carcinoma of the thyroid a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978;41:2267–2275. [PubMed: 657091]
- Schwartz DL, Lobo ML, Ang KK, et al. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys. 2009;75:1–9. [PubMed: 19095376]
- Rao SN, Cabanillas ME. Navigating systemic therapy in advanced thyroid carcinoma: From standard care to personalized therapy and beyond. J Endocr Soc. 2018 Oct 1;2(10):1109–1130. [PubMed: 30250937]
- Kebebew E, Ituarte PHG, Siperstein AE, et al. Medullary thyroid carcinoma clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139–1147. [PubMed]
- Saad MF, Ordonez NG, Rashid RK, et al. Medullary carcinoma of the thyroid: a study of the clinical features and prognostic factors in 161 patients. Medicine (Baltimore). 1984;63:319. [PubMed]
- Scopsi L, Sampietro G, Boracchi P, et al. Multivariate analysis of prognostic factors in sporadic medullary carcinoma of the thyroid: a retrospective study of 109 consecutive patients. Cancer. 1996;78:2173–2183. [PubMed]
- deGroot JW, Plukker JT, Wolffenbuttel BH, et al. Determinants of life expectancy in medullary thyroid cancer: age does not matter. Clin Endocrinol (Oxf). 2006;65:729. [PubMed]
- Machens A, Hauptmann S, Dralle H. Increased risk of lymph node metastasis in multifocal hereditary and sporadic medullary thyroid cancer. World J Surg. 2007;31:1960–1965. [PubMed]
- Roy M, Chen H, Sippel RS. Current understanding and management of medullary thyroid cancer. The Oncologist. 2013;18:1093–1100. [PubMed]
- Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double blind phase III trial. J Clin Oncol. 2012;30:134. [PubMed]
- Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013 Oct 10;31(29):3639–46. [PubMed]