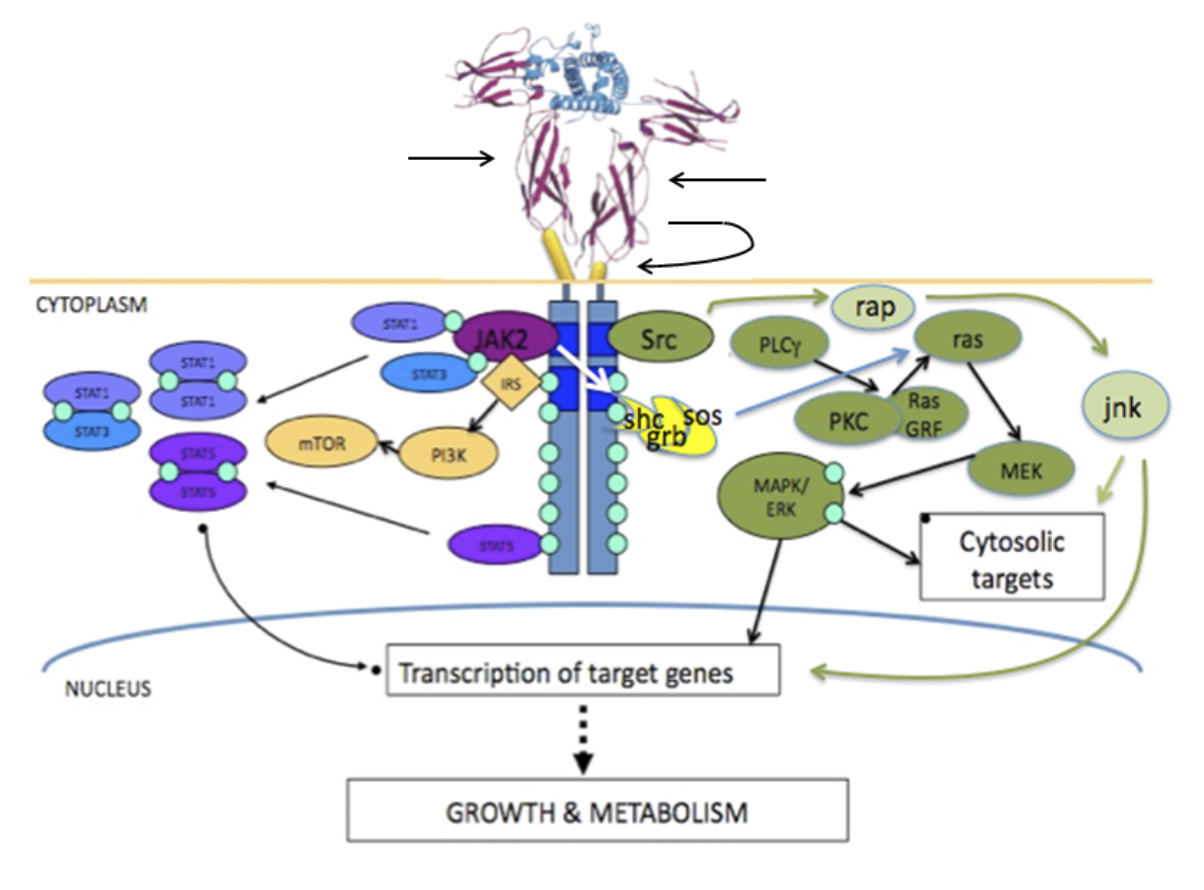
Figure 2 – Growth hormone binding to the extracellular domain of the growth hormone receptor reorients the pre-existing homodimer so that one growth hormone receptor subunit rotates relative to the other. This structural reorientation is transmitted through the transmembrane domain resulting in a repositioning of tyrosine kinases bound to the cytoplasmic domain of the receptors. The distance between the box 1 motifs increases between inactive and active states and this movement is fundamental to activation of JAK2. Phosphorylation of JAK2 in turn leads to phosphorylation of STAT molecules, activation of the MAPK cascade and activation of IRS-1. STAT5a and STAT5b homo/heterodimerise and translocate to the nucleus. Figure kindly supplied by Dr Andrew Brooks, Institute for Molecular Bioscience, The University of Queensland.
