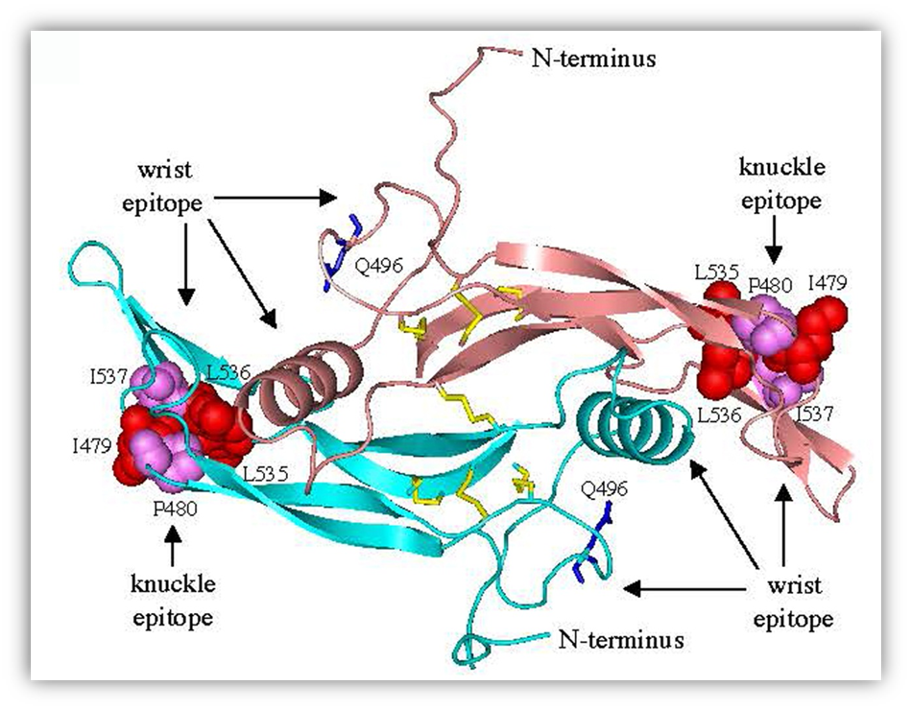
FIGURE 20. Molecular model of C-terminal AMH. A three-dimensional model of the C-terminal dimer was generated by comparative modeling using human BMP9 (399) as a template. The wrist epitope, the putative binding site for the type I receptor, is composed of the prehelix loop and alpha-helix of one monomer together with the concave side of the fingers of the second monomer (400). A mutation in the prehelix loop of AMH, Q496H, causes persistent Müllerian duct syndrome (397). Residues in the knuckle epitope of AMH, the putative binding site for AMHR2, are similar to those present in BMP7 and activin at the interface with ACTR2B (401, 402). Disulfide bonds (yellow) and Q496 residues (blue) are shown as sticks; residues in the knuckle epitopes are shown as spheres. Reprinted from ref. (397): Belville C, Van Vlijmen H, Ehrenfels C, Pepinsky RB, Rezaie AR, Picard J, Josso N, di Clemente N, Cate RL. Mutations of the anti-Müllerian hormone gene in patients with persistent Müllerian duct syndrome: biosynthesis, secretion and processing of the abnormal proteins and analysis using a three-dimensional model. Molecular Endocrinology 18:708-721 (2004). Copyright 2004 The Endocrine Society with permission. http://mend.endojournals.org/content/18/3/708.abstract?sid=22a37d21-69b5-499e-9996-8b1d4df81215
